
SL Paper 2
Consider the following equilibrium.
\[\begin{array}{*{20}{l}} {{\text{2S}}{{\text{O}}_2}{\text{(g)}} + {{\text{O}}_2}{\text{(g)}} \rightleftharpoons {\text{2S}}{{\text{O}}_3}{\text{(g)}}}&{\Delta {H^\Theta } = - 198{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \end{array}\]
Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.
State and explain the effect of increasing the temperature on the yield of sulfur trioxide.
State the effect of a catalyst on the value of \({K_{\text{c}}}\).
State and explain the effect of a catalyst on the position of equilibrium.
Define oxidation in terms of oxidation numbers.
Describe using a labelled diagram, the essential components of an electrolytic cell.
Explain why solid sodium chloride does not conduct electricity but molten sodium chloride does.
Molten sodium chloride undergoes electrolysis in an electrolytic cell. For each electrode deduce the half-equation and state whether oxidation or reduction takes place. Deduce the equation of the overall cell reaction including state symbols.
Electrolysis has made it possible to obtain reactive metals such as aluminium from their ores, which has resulted in significant developments in engineering and technology. State one reason why aluminium is preferred to iron in many uses.
Outline two differences between an electrolytic cell and a voltaic cell.
To determine the enthalpy change of combustion of methanol, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\), 0.230 g of methanol was combusted in a spirit burner. The heat released increased the temperature of \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of water from 24.5 °C to 45.8 °C.
The manufacture of gaseous methanol from CO and \({{\text{H}}_{\text{2}}}\) involves an equilibrium reaction.
\[{\text{CO(g)}} + {\text{2}}{{\text{H}}_2}{\text{(g)}} \rightleftharpoons {\text{C}}{{\text{H}}_3}{\text{OH(g)}}\,\,\,\,\,\Delta {H^\Theta } < 0\]
State and explain the effect of the following changes on the equilibrium position of the reaction in part (c).
Calculate the enthalpy change of combustion of methanol.
Using the theoretical value in Table 12 of the Data Booklet, discuss the experimental results, including one improvement that could be made.
Methanol can be produced according to the following equation.
\[{\text{CO(g)}} + {\text{2}}{{\text{H}}_2}{\text{(g)}} \to {\text{C}}{{\text{H}}_3}{\text{OH(l)}}\]
Calculate the standard enthalpy change of this reaction using the following data:
\[\begin{array}{*{20}{l}} {{\text{I: 2C}}{{\text{H}}_3}{\text{OH(l)}} + {\text{3}}{{\text{O}}_2}{\text{(g)}} \to {\text{2C}}{{\text{O}}_2}{\text{(g)}} + {\text{4}}{{\text{H}}_{\text{2}}}{\text{O(l)}}}&{\Delta {H^\Theta } = - 1452{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \\ {{\text{II: 2CO(g)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{2C}}{{\text{O}}_2}{\text{(g)}}}&{\Delta {H^\Theta } = - 566{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \\ {{\text{III: 2}}{{\text{H}}_2}{\text{(g)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{2}}{{\text{H}}_2}{\text{O(l)}}}&{\Delta {H^\Theta } = - 572{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \end{array}\]
Outline the characteristics of a chemical equilibrium.
Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for this reaction.
Increase in temperature.
Increase in pressure.
Addition of a catalyst.
Biodiesel makes use of plants’ ability to fix atmospheric carbon by photosynthesis. Many companies and individuals are now using biodiesel as a fuel in order to reduce their carbon footprint. Biodiesel can be synthesized from vegetable oil according to the following reaction.

The reversible arrows in the equation indicate that the production of biodiesel is an equilibrium process.
Identify the organic functional group present in both vegetable oil and biodiesel.
For part of her extended essay investigation into the efficiency of the process, a student reacted a pure sample of a vegetable oil (where \({\text{R}} = {{\text{C}}_{{\text{17}}}}{{\text{H}}_{{\text{33}}}}\)) with methanol. The raw data recorded for the reaction is below.
\[\begin{array}{*{20}{l}} {{\text{Mass of oil}}}&{ = 1013.0{\text{ g}}} \\ {{\text{Mass of methanol}}}&{ = 200.0{\text{ g}}} \\ {{\text{Mass of sodium hydroxide}}}&{ = 3.5{\text{ g}}} \\ {{\text{Mass of biodiesel produced}}}&{ = 811.0{\text{ g}}} \end{array}\]
The relative molecular mass of the oil used by the student is 885.6. Calculate the amount (in moles) of the oil and the methanol used, and hence the amount (in moles) of excess methanol.
State what is meant by the term dynamic equilibrium.
Using the abbreviations [vegetable oil], [methanol], [glycerol] and [biodiesel] deduce the equilibrium constant expression \({\text{(}}{K_{\text{c}}}{\text{)}}\) for this reaction.
Suggest a reason why excess methanol is used in this process.
State and explain the effect that the addition of the sodium hydroxide catalyst will have on the position of equilibrium.
The reactants had to be stirred vigorously because they formed two distinct layers in the reaction vessel. Explain why they form two distinct layers and why stirring increases the rate of reaction.
Calculate the percentage yield of biodiesel obtained in this process.
Methanol may be produced by the exothermic reaction of carbon monoxide gas and hydrogen gas.
\[\begin{array}{*{20}{l}} {{\text{CO(g)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH(g)}}}&{\Delta {H^\Theta } = - 103{\text{ kJ}}} \end{array}\]
State and explain the effect of changing the following conditions on the amount of methanol present at equilibrium:
State the equilibrium constant expression, \({K_{\text{c}}}\), for the production of methanol.
increasing the temperature of the reaction at constant pressure.
increasing the pressure of the reaction at constant temperature.
The conditions used in industry during the production of methanol are a temperature of 450 °C and pressure of up to 220 atm. Explain why these conditions are used rather than those that could give an even greater amount of methanol.
A catalyst of copper mixed with zinc oxide and alumina is used in industry for this production of methanol. Explain the function of the catalyst.
An example of a homogeneous reversible reaction is the reaction between hydrogen and iodine.
\[{{\text{H}}_{\text{2}}}{\text{(g)}} + {{\text{I}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2HI(g)}}\]
Propane can be formed by the hydrogenation of propene.
\[{\text{C}}{{\text{H}}_3}{\text{CH=C}}{{\text{H}}_2}{\text{(g)}} + {{\text{H}}_2}{\text{(g)}} \to {\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}{\text{(g)}}\]
Outline the characteristics of a homogeneous chemical system that is in a state of equilibrium.
Deduce the expression for the equilibrium constant, \({K_{\text{c}}}\).
Predict what would happen to the position of equilibrium and the value of \({K_{\text{c}}}\) if the pressure is increased from 1 atm to 2 atm.
The value of \({K_{\text{c}}}\) at 500 K is 160 and the value of \({K_{\text{c}}}\) at 700 K is 54. Deduce what this information tells us about the enthalpy change of the forward reaction.
The reaction can be catalysed by adding platinum metal. State and explain what effect the addition of platinum would have on the value of the equilibrium constant.
State the conditions necessary for the hydrogenation reaction to occur.
Enthalpy changes can be determined using average bond enthalpies. Define the term average bond enthalpy.
Determine a value for the hydrogenation of propene using information from Table 10 of the Data Booklet.
Explain why the enthalpy of hydrogenation of propene is an exothermic process.
Describe a chemical test that could be used to distinguish between propane and propene. In each case state the result of the test.
Under certain conditions propene can polymerize to form poly(propene). State the type of polymerization taking place and draw a section of the polymer to represent the repeating unit.
Other than polymerization, state one reaction of alkenes which is of economic importance.
The Contact process involves an exothermic reversible reaction.
\({\text{2S}}{{\text{O}}_{\text{2}}}{\text{(g)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2S}}{{\text{O}}_{\text{3}}}{\text{(g)}}\) \({K_{\text{c}}} \gg 1\) at 200 °C and 1 atm
Deduce the extent of the reaction at 200 °C and 1 atm.
The Contact process operates at a temperature of 450 °C and a pressure of 2 atm as optimum conditions for the production of \({\text{S}}{{\text{O}}_{\text{3}}}\). Outline the reasons for choosing these conditions.
Temperature:
Pressure:
An engineer at a Contact process plant hypothesized that using pure oxygen, instead of air, would increase the profits. Comment on whether or not her hypothesis is valid, giving your reasons.
When nitrogen gas and hydrogen gas are allowed to react in a closed container, the following equilibrium is established.
\[{{\text{N}}_{\text{2}}}{\text{(g)}} + {\text{3}}{{\text{H}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2N}}{{\text{H}}_{\text{3}}}{\text{(g)}}\;\;\;\;\;\Delta H = - 92.6{\text{ kJ}}\]
Outline two characteristics of a reversible reaction in a state of dynamic equilibrium.
Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.
Predict, with a reason, how each of the following changes affects the position of equilibrium.
The volume of the container is increased.
Ammonia is removed from the equilibrium mixture.
Define the term activation energy, \({E_{\text{a}}}\).
Ammonia is manufactured by the Haber process in which iron is used as a catalyst. Explain the effect of a catalyst on the rate of reaction.
Sketch the Maxwell–Boltzmann energy distribution curve for a reaction, labelling both axes and showing the activation energy with and without a catalyst.
Typical conditions used in the Haber process are 500 °C and 200 atm, resulting in approximately 15% yield of ammonia.
(i) Explain why a temperature lower than 500 °C is not used.
(ii) Outline why a pressure higher than 200 atm is not often used.
Define the term base according to the Lewis theory.
Define the term weak base according to the Brønsted-Lowry theory.
Deduce the formulas of conjugate acid-base pairs in the reaction below.
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{N}}{{\text{H}}_{\text{2}}}{\text{(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}} \rightleftharpoons {\text{C}}{{\text{H}}_{\text{3}}}{\text{NH}}_{\text{3}}^ + {\text{(aq)}} + {\text{O}}{{\text{H}}^ - }{\text{(aq)}}\]

Outline an experiment and its results which could be used to distinguish between a strong base and a weak base.
Group 7 of the periodic table contains a number of reactive elements such as chlorine, bromine and iodine.
Bleaches in which chlorine is the active ingredient are the most common, although some environmental groups have concerns about their use. In aqueous chlorine the equilibrium below produces chloric(I) acid (hypochlorous acid), HOCl, the active bleach.
\[{\text{C}}{{\text{l}}_2}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {\text{HOCl (aq)}} + {{\text{H}}^ + }{\text{(aq)}} + {\text{C}}{{\text{l}}^ - }{\text{(aq)}}\]
Aqueous sodium chlorate(I), NaOCl, the most common active ingredient in chlorine based bleaches, oxidizes coloured materials to colourless products while being reduced to the chloride ion. It will also oxidize sulfur dioxide to the sulfate ion.
(i) Describe the colour change that occurs when aqueous chlorine is added to aqueous sodium bromide.
(ii) Outline, with the help of a chemical equation, why this reaction occurs.
The colour change in the reaction between aqueous chlorine and aqueous sodium iodide is very similar, but it differs with an excess of aqueous chlorine. Describe the appearance of the reaction mixture when excess aqueous chlorine has been added to aqueous sodium iodide.
Chloric(I) acid is a weak acid, but hydrochloric acid is a strong acid. Outline how this is indicated in the equation above.
State a balanced equation for the reaction of chloric(I) acid with water.
Outline, in terms of the equilibrium above, why it is dangerous to use an acidic toilet cleaner in combination with this kind of bleach.
Suggest why a covalent molecule, such as chloric(I) acid, is readily soluble in water.
Draw the Lewis (electron dot) structure of chloric(I) acid.
Predict the H–O–Cl bond angle in this molecule and explain this in terms of the valence shell electron pair repulsion (VSEPR) theory.
(i) Deduce the coefficients required to balance the half-equations given below.
___ \({\text{Cl}}{{\text{O}}^ - } + \) ___ \({{\text{H}}^ + } + \) ___ \({{\text{e}}^ - } \rightleftharpoons \) ___ \({{\text{H}}_2}{\text{O}} + \) ___ \({\text{C}}{{\text{l}}^ - }\)
___ \({\text{SO}}_4^{2 - }\) ___ \({{\text{H}}^ + } + \) ___ \({{\text{e}}^ - } \rightleftharpoons \) ___ \({\text{S}}{{\text{O}}_2} + \) ___ \({{\text{H}}_2}{\text{O}}\)
(ii) State the initial and final oxidation numbers of both chlorine and sulfur in the equations in part (i).

(iii) Use the half-equations to deduce the balanced equation for the reaction between the chlorate(I) ion and sulfur dioxide.
Ethane-1,2-diol, HOCH2CH2OH, has a wide variety of uses including the removal of ice from aircraft and heat transfer in a solar cell.
Ethane-1,2-diol can be formed according to the following reaction.
2CO (g) + 3H2 (g) \( \rightleftharpoons \) HOCH2CH2OH (g)
(i) Deduce the equilibrium constant expression, Kc, for this reaction.
(ii) State how increasing the pressure of the reaction mixture at constant temperature will affect the position of equilibrium and the value of Kc.
Position of equilibrium:
Kc:
(iii) Calculate the enthalpy change, ΔHθ, in kJ, for this reaction using section 11 of the data booklet. The bond enthalpy of the carbon–oxygen bond in CO (g) is 1077kJmol-1.
(iv) The enthalpy change, ΔHθ, for the following similar reaction is –233.8 kJ.
2CO(g) + 3H2(g) \( \rightleftharpoons \) HOCH2CH2OH (l)
Deduce why this value differs from your answer to (a)(iii).
Determine the average oxidation state of carbon in ethene and in ethane-1,2-diol.
Ethene:
Ethane-1,2-diol:
Explain why the boiling point of ethane-1,2-diol is significantly greater than that of ethene.
Ethane-1,2-diol can be oxidized first to ethanedioic acid, (COOH)2, and then to carbon dioxide and water. Suggest the reagents to oxidize ethane-1,2-diol.
Consider the following equilibrium:
\[\begin{array}{*{20}{l}} {{\text{4N}}{{\text{H}}_3}{\text{(g)}} + {\text{5}}{{\text{O}}_2}{\text{(g)}} \rightleftharpoons {\text{4NO(g)}} + {\text{6}}{{\text{H}}_2}{\text{O(g)}}}&{\Delta {H^\Theta } = - 909{\text{ kJ}}} \end{array}\]
Nitrogen reacts with hydrogen to form ammonia in the Haber process, according to the following equilibrium.
\[\begin{array}{*{20}{l}} {{{\text{N}}_{\text{2}}}{\text{(g)}} + {\text{3}}{{\text{H}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2N}}{{\text{H}}_{\text{3}}}{\text{(g)}}}&{\Delta {H^\Theta } = - 92.6{\text{ kJ}}} \end{array}\]
Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.
Predict the direction in which the equilibrium will shift when the following changes occur.
The volume increases.
The temperature decreases.
\({{\text{H}}_{\text{2}}}{\text{O(g)}}\) is removed from the system.
A catalyst is added to the reaction mixture.
Define the term activation energy, \({E_{\text{a}}}\).
Nitrogen monoxide, NO, is involved in the decomposition of ozone according to the following mechanism.
\[\begin{array}{*{20}{l}} {}&{{{\text{O}}_{\text{3}}} \to {{\text{O}}_{\text{2}}} + {\text{O}} \bullet } \\ {}&{{{\text{O}}_3} + {\text{NO}} \to {\text{N}}{{\text{O}}_2} + {{\text{O}}_2}} \\ {}&{{\text{N}}{{\text{O}}_2} + {\text{O}} \bullet \to {\text{NO}} + {{\text{O}}_2}} \\ {{\text{Overall:}}}&{{\text{2}}{{\text{O}}_3} \to {\text{3}}{{\text{O}}_2}} \end{array}\]
State and explain whether or not NO is acting as a catalyst.
Define the term endothermic reaction.
Sketch the Maxwell-Boltzmann energy distribution curve for a reaction with and without a catalyst, and label both axes.
Define the term rate of reaction.
Iron, used as the catalyst in the Haber process, has a specific heat capacity of \({\text{0.4490 J}}\,{{\text{g}}^{ - 1}}{{\text{K}}^{ - 1}}\). If 245.0 kJ of heat is supplied to 8.500 kg of iron, initially at a temperature of 15.25 °C, determine its final temperature in K.
Consider the following three redox reactions.
\[\begin{array}{*{20}{l}} {{\text{Cd(s)}} + {\text{N}}{{\text{i}}^{2 + }}{\text{(aq)}} \to {\text{C}}{{\text{d}}^{2 + }}{\text{(aq)}} + {\text{Ni(s)}}} \\ {{\text{Ni(s)}} + {\text{2A}}{{\text{g}}^ + }{\text{(aq)}} \to {\text{N}}{{\text{i}}^{2 + }}{\text{(aq)}} + {\text{2Ag(s)}}} \\ {{\text{Zn(s)}} + {\text{C}}{{\text{d}}^{2 + }}{\text{(aq)}} \to {\text{Z}}{{\text{n}}^{2 + }}{\text{(aq)}} + {\text{Cd(s)}}} \end{array}\]
(i) Draw an annotated diagram of a voltaic cell composed of a magnesium electrode in \({\text{1.0 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) magnesium nitrate solution and a silver electrode in \({\text{1.0 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) silver nitrate solution. State the direction of electron flow on your diagram.
(ii) Deduce half-equations for the oxidation and reduction reactions.
(i) Deduce the order of reactivity of the four metals, cadmium, nickel, silver and zinc and list in order of decreasing reactivity.
(ii) Identify the best oxidizing agent and the best reducing agent.
(i) Solid sodium chloride does not conduct electricity but molten sodium chloride does. Explain this difference.
(ii) Outline what happens in an electrolytic cell during the electrolysis of molten sodium chloride using inert electrodes. Deduce equations for the reactions occurring at each electrode.
(i) A state of equilibrium can exist when a piece of copper metal is placed in a solution of copper(II) sulfate. Outline the characteristics of a chemical system in dynamic equilibrium.
(ii) For an exothermic reaction state how an increase in temperature would affect both \({K_{\text{c}}}\) and the position of equilibrium.
Consider the following reaction taking place at 375 °C in a \({\text{1.00 d}}{{\text{m}}^{\text{3}}}\) closed container.
\[\begin{array}{*{20}{l}} {{\text{C}}{{\text{l}}_{\text{2}}}{\text{(g)}} + {\text{S}}{{\text{O}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}{\text{(g)}}}&{\Delta {H^\Theta } = - 84.5{\text{ kJ}}} \end{array}\]
A solution of hydrogen peroxide, \({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\), is added to a solution of sodium iodide, NaI, acidified with hydrochloric acid, HCl. The yellow colour of the iodine, \({{\text{I}}_{\text{2}}}\), can be used to determine the rate of reaction.
\[{{\text{H}}_2}{{\text{O}}_2}{\text{(aq)}} + {\text{2NaI(aq)}} + {\text{2HCl(aq)}} \to {\text{2NaCl(aq)}} + {{\text{I}}_2}{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}\]
The experiment is repeated with some changes to the reaction conditions. For each of the changes that follow, predict, stating a reason, its effect on the rate of reaction.
Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.
If the temperature of the reaction is changed to 300 °C, predict, stating a reason in each case, whether the equilibrium concentration of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\) and the value of \({K_{\text{c}}}\) will increase or decrease.
If the volume of the container is changed to \({\text{1.50 d}}{{\text{m}}^{\text{3}}}\), predict, stating a reason in each case, how this will affect the equilibrium concentration of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\) and the value of \({K_{\text{c}}}\).
Suggest, stating a reason, how the addition of a catalyst at constant pressure and temperature will affect the equilibrium concentration of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\).
Graphing is an important method in the study of the rates of chemical reaction. Sketch a graph to show how the reactant concentration changes with time in a typical chemical reaction taking place in solution. Show how the rate of the reaction at a particular time can be determined.
The concentration of \({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\) is increased at constant temperature.
The solution of NaI is prepared from a fine powder instead of large crystals.
Explain why the rate of a reaction increases when the temperature of the system increases.
An equilibrium occurs between nitrogen dioxide, \({\text{N}}{{\text{O}}_{\text{2}}}{\text{(g)}}\), and dinitrogen tetroxide, \({{\text{N}}_{\text{2}}}{{\text{O}}_{\text{4}}}{\text{(g)}}\).
\({\text{2N}}{{\text{O}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {{\text{N}}_{\text{2}}}{{\text{O}}_{\text{4}}}{\text{(g)}}\) \(\Delta H = - 58{\text{ kJ}}\)
Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for this reaction.
Explain the effect on the position of the equilibrium and on the value of \({K_{\text{c}}}\) when:
(i) pressure is decreased and temperature is kept constant.
(ii) temperature is increased and pressure is kept constant.
The Haber process enables the large-scale production of ammonia needed to make fertilizers.
The equation for the Haber process is given below.
\[{{\text{N}}_2}({\text{g)}} + 3{{\text{H}}_2}({\text{g)}} \rightleftharpoons {\text{2N}}{{\text{H}}_3}({\text{g)}}\]
The percentage of ammonia in the equilibrium mixture varies with temperature.

Fertilizers may cause health problems for babies because nitrates can change into nitrites in water used for drinking.
A student decided to investigate the reactions of the two acids with separate samples of \({\text{0.20 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution.
(i) Use the graph to deduce whether the forward reaction is exothermic or endothermic and explain your choice.
(ii) State and explain the effect of increasing the pressure on the yield of ammonia.
(iii) Explain the effect of increasing the temperature on the rate of reaction.
(i) Define oxidation in terms of oxidation numbers.
(ii) Deduce the oxidation states of nitrogen in the nitrate, \({\text{NO}}_{\text{3}}^ - \), and nitrite, \({\text{NO}}_{\text{2}}^ - \), ions.
The nitrite ion is present in nitrous acid, HNO2, which is a weak acid. The nitrate ion is present in nitric acid, HNO3, which is a strong acid. Distinguish between the terms strong and weak acid and state the equations used to show the dissociation of each acid in aqueous solution.
A small piece of magnesium ribbon is added to solutions of nitric and nitrous acid of the same concentration at the same temperature. Describe two observations that would allow you to distinguish between the two acids.
(i) Calculate the volume of the sodium hydroxide solution required to react exactly with a \({\text{15.0 c}}{{\text{m}}^{\text{3}}}\) solution of \({\text{0.10 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) nitric acid.
(ii) The following hypothesis was suggested by the student: “Since nitrous acid is a weak acid it will react with a smaller volume of the \({\text{0.20 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution.” Comment on whether or not this is a valid hypothesis.
The graph below shows how the conductivity of the two acids changes with concentration.

Identify Acid 1 and explain your choice.
Nitric acid reacts with silver in a redox reaction.
__ \({\text{Ag(s)}} + \) __ \({\text{NO}}_3^ - {\text{(aq)}} + \) ___ \( \to \) ___\({\text{A}}{{\text{g}}^ + }{\text{(aq)}} + \) __ \({\text{NO(g)}} + \) ____
Using oxidation numbers, deduce the complete balanced equation for the reaction showing all the reactants and products.
A class studied the equilibrium established when ethanoic acid and ethanol react together in the presence of a strong acid, using propanone as an inert solvent. The equation is given below.
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}} + {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH}} \rightleftharpoons {\text{C}}{{\text{H}}_{\text{3}}}{\text{COO}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}} + {{\text{H}}_{\text{2}}}{\text{O}}\]
One group made the following initial mixture:

After one week, a \(5.00 \pm 0.05{\text{ c}}{{\text{m}}^{\text{3}}}\) sample of the final equilibrium mixture was pipetted out and titrated with \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 2}}\) aqueous sodium hydroxide to determine the amount of ethanoic acid remaining. The following titration results were obtained:

The density of ethanoic acid is \({\text{1.05 g}}\,{\text{c}}{{\text{m}}^{ - 3}}\). Determine the amount, in mol, of ethanoic acid present in the initial mixture.
The hydrochloric acid does not appear in the balanced equation for the reaction. State its function.
Identify the liquid whose volume has the greatest percentage uncertainty.
(i) Calculate the absolute uncertainty of the titre for Titration 1 (\({\text{27.60 c}}{{\text{m}}^{\text{3}}}\)).
(ii) Suggest the average volume of alkali, required to neutralize the \({\text{5.00 c}}{{\text{m}}^{\text{3}}}\) sample, that the student should use.
(iii) \({\text{23.00 c}}{{\text{m}}^{\text{3}}}\) of this \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) aqueous sodium hydroxide reacted with the ethanoic acid in the \({\text{5.00 c}}{{\text{m}}^{\text{3}}}\) sample. Determine the amount, in mol, of ethanoic acid present in the \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of final equilibrium mixture.
Referring back to your answer for part (a), calculate the percentage of ethanoic acid converted to ethyl ethanoate.
Deduce the equilibrium constant expression for the reaction.
Outline how you could establish that the system had reached equilibrium at the end of one week.
Outline why changing the temperature has only a very small effect on the value of the equilibrium constant for this equilibrium.
Outline how adding some ethyl ethanoate to the initial mixture would affect the amount of ethanoic acid converted to product.
Propanone is used as the solvent because one compound involved in the equilibrium is insoluble in water. Identify this compound and explain why it is insoluble in water.
Suggest one other reason why using water as a solvent would make the experiment less successful.
In acidic solution, ions containing titanium can react according to the half-equation below.
\[{\text{Ti}}{{\text{O}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {{\text{e}}^ - } \rightleftharpoons {\text{T}}{{\text{i}}^{3 + }}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}}\]
A reactivity series comparing titanium, cadmium and europium is given below.
Least reactive Cd \( < \) Ti \( < \) Eu Most reactive
The half-equations corresponding to these metals are:
\({\text{E}}{{\text{u}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \rightleftharpoons {\text{Eu(s)}}\)
\({\text{T}}{{\text{i}}^{3 + }}{\text{(aq)}} + {\text{3}}{{\text{e}}^ - } \rightleftharpoons {\text{Ti(s)}}\)
\({\text{C}}{{\text{d}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \rightleftharpoons {\text{Cd(s)}}\)
Some students were provided with a \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of a monobasic acid, HQ, and given the problem of determining whether HQ was a weak acid or a strong acid.
State the initial and final oxidation numbers of titanium and hence deduce whether it is oxidized or reduced in this change.

Considering the above equilibrium, predict, giving a reason, how adding more acid would affect the strength of the \({\text{Ti}}{{\text{O}}^{2 + }}\) ion as an oxidizing agent.
Deduce which of the species would react with titanium metal.
Deduce the balanced equation for this reaction.
Deduce which of the six species is the strongest oxidizing agent.
A voltaic cell can be constructed using cadmium and europium half-cells. State how the two solutions involved should be connected and outline how this connection works.
Define a Brønsted–Lowry acid.
Distinguish between the terms strong acid and weak acid.
Neelu and Charles decided to solve the problem by determining the volume of \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution needed to neutralize \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of the acid. Outline whether this was a good choice.
Neelu and Charles decided to compare the volume of sodium hydroxide solution needed with those required by known \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) strong and weak acids. Unfortunately they chose sulfuric acid as the strong acid. Outline why this was an unsuitable choice.
State a suitable choice for both the strong acid and the weak acid.
Strong acid:
Weak acid:
Francisco and Shamiso decided to measure the pH of the initial solution, HQ, and they found that its pH was 3.7. Deduce, giving a reason, the strength (weak or strong) of the acid HQ.
Suggest a method, other than those mentioned above, that could be used to solve the problem and outline how the results would distinguish between a strong acid and a weak acid.
A hydrocarbon has the empirical formula \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{7}}}\). When 1.17 g of the compound is heated to 85 °C at a pressure of 101 kPa it occupies a volume of \({\text{400 c}}{{\text{m}}^{\text{3}}}\).
(i) Calculate the molar mass of the compound, showing your working.
(ii) Deduce the molecular formula of the compound.
\({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}\) exists as three isomers. Identify the structure of the isomer with the lowest boiling point and explain your choice.
Ethanol is a primary alcohol that can be oxidized by acidified potassium dichromate(VI). Distinguish between the reaction conditions needed to produce ethanal and ethanoic acid.
Ethanal:
Ethanoic acid:
Determine the oxidation number of carbon in ethanol and ethanal.
Ethanol:
Ethanal:
Deduce the half-equation for the oxidation of ethanol to ethanal.
Deduce the overall redox equation for the reaction of ethanol to ethanal with acidified potassium dichromate(VI) by combining your answer to part (c) (iii) with the following half-equation:
\[{\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{2 - }{\text{(aq)}} + {\text{14}}{{\text{H}}^ + }{\text{(aq)}} + {\text{6}}{{\text{e}}^ - } \to {\text{2C}}{{\text{r}}^{3 + }}{\text{(aq)}} + {\text{7}}{{\text{H}}_{\text{2}}}{\text{O(l)}}\]
Describe two characteristics of a reaction at equilibrium.
Describe how a catalyst increases the rate of a reaction.
State and explain the effect of a catalyst on the position of equilibrium.
Ethanoic acid reacts with ethanol to form the ester ethyl ethanoate.
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH(l)}} + {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH(l)?????C}}{{\text{H}}_{\text{3}}}{\text{COOC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}{\text{(l)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}}\]
The esterification reaction is exothermic. State the effect of increasing temperature on the value of the equilibrium constant (\({K_{\text{c}}}\)) for this reaction.
Urea, (H2N)2CO, is excreted by mammals and can be used as a fertilizer.
Calculate the percentage by mass of nitrogen in urea to two decimal places using section 6 of the data booklet.
Suggest how the percentage of nitrogen affects the cost of transport of fertilizers giving a reason.
The structural formula of urea is shown.

Predict the electron domain and molecular geometries at the nitrogen and carbon atoms, applying the VSEPR theory.

Urea can be made by reacting potassium cyanate, KNCO, with ammonium chloride, NH4Cl.
KNCO(aq) + NH4Cl(aq) → (H2N)2CO(aq) + KCl(aq)
Determine the maximum mass of urea that could be formed from 50.0 cm3 of 0.100 mol dm−3 potassium cyanate solution.
Urea can also be made by the direct combination of ammonia and carbon dioxide gases.
2NH3(g) + CO2(g) \( \rightleftharpoons \) (H2N)2CO(g) + H2O(g) ΔH < 0
Predict, with a reason, the effect on the equilibrium constant, Kc, when the temperature is increased.
Suggest one reason why urea is a solid and ammonia a gas at room temperature.
Sketch two different hydrogen bonding interactions between ammonia and water.
The combustion of urea produces water, carbon dioxide and nitrogen.
Formulate a balanced equation for the reaction.
The mass spectrum of urea is shown below.

Identify the species responsible for the peaks at m/z = 60 and 44.
The IR spectrum of urea is shown below.

Identify the bonds causing the absorptions at 3450 cm−1 and 1700 cm−1 using section 26 of the data booklet.
Predict the number of signals in the 1H NMR spectrum of urea.
Water is an important substance that is abundant on the Earth’s surface. Water dissociates according to the following equation.
\[{{\text{H}}_{\text{2}}}{\text{O(l)}} \rightleftharpoons {{\text{H}}^ + }{\text{(aq)}} + {\text{O}}{{\text{H}}^ - }{\text{(aq)}}\]
The graph below shows how the volume of carbon dioxide formed varies with time when a hydrochloric acid solution is added to excess calcium carbonate in a flask.

(i) State the equilibrium constant expression for the dissociation of water.
(ii) Explain why even a very acidic aqueous solution still has some \({\text{O}}{{\text{H}}^ - }\) ions present in it.
(iii) State and explain the effect of increasing temperature on the equilibrium constant above given that the dissociation of water is an endothermic process.
(iv) The pH of a solution is 2. If its pH is increased to 6, deduce how the hydrogen ion concentration changes.
In carbonated drinks containing dissolved carbon dioxide under high pressure, the
following dynamic equilibrium exists.
\[{\text{C}}{{\text{O}}_2}({\text{aq)}} \rightleftharpoons {\text{C}}{{\text{O}}_2}({\text{g)}}\]
Describe the effect of opening a carbonated drink container and outline how this
equilibrium is affected.
(i) Explain the shape of the curve.
(ii) Copy the above graph on your answer sheet and sketch the curve you would obtain if double the volume of hydrochloric acid solution of half the concentration as in the example above is used instead, with all other variables kept constant from the original. Explain why the shape of the curve is different.
(iii) Outline one other way in which the rate of this reaction can be studied in a school laboratory. Sketch a graph to illustrate how the selected variable would change with time.
(iv) Define the term activation energy and state one reason why the reaction between calcium carbonate and hydrochloric acid takes place at a reasonably fast rate at room temperature.
A mixture of 1.00 mol SO2(g), 2.00 mol O2(g) and 1.00 mol SO3(g) is placed in a 1.00 dm3 container and allowed to reach equilibrium.
2SO2(g) + O2(g) \( \rightleftharpoons \) 2SO3(g)
Distinguish between the terms reaction quotient, Q, and equilibrium constant, Kc.
The equilibrium constant, Kc, is 0.282 at temperature T.
Deduce, showing your work, the direction of the initial reaction.
Chemical equilibrium and kinetics are important concepts in chemistry.
The oxidation of sulfur dioxide is an important reaction in the Contact process used to manufacture sulfuric acid.
\[\begin{array}{*{20}{l}} {{\text{2S}}{{\text{O}}_2}{\text{(g)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2S}}{{\text{O}}_{\text{3}}}{\text{(g)}}}&{\Delta H = - 198.2{\text{ kJ}}} \end{array}\]
Vanadium(V) oxide, \({{\text{V}}_{\text{2}}}{{\text{O}}_{\text{5}}}\), is a catalyst that can be used in the Contact process. It provides an alternative pathway for the reaction, lowering the activation energy, \({E_{\text{a}}}\).
A glass container is half-filled with liquid bromine and then sealed. The system eventually reaches a dynamic equilibrium. State one characteristic of a system in equilibrium.
(i) Deduce the equilibrium constant expression, \({K_{\text{c}}}\).
(ii) Predict how each of the following changes affects the position of equilibrium and
the value of \({K_{\text{c}}}\).

(i) Define the term activation energy, \({E_{\text{a}}}\).
(ii) Sketch the two Maxwell–Boltzmann energy distribution curves for a fixed amount of gas at two different temperatures, \({T_1}\) and \({T_2}{\text{ }}({T_2} > {T_1})\). Label both axes.

PCl5(g) and Cl2(g) were placed in a sealed flask and allowed to reach equilibrium at 200 °C. The enthalpy change, ΔH, for the decomposition of PCl5(g) is positive.

Deduce the equilibrium constant expression, Kc, for the decomposition of PCl5(g).
Deduce, giving a reason, the factor responsible for establishing the new equilibrium after 14 minutes.
Deduce the Lewis (electron dot) structure and molecular geometry of PCl3.
Two hydrides of nitrogen are ammonia and hydrazine, N2H4. One derivative of ammonia is methanamine whose molecular structure is shown below.
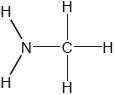
Hydrazine is used to remove oxygen from water used to generate steam or hot water.
N2H4(aq) + O2(aq) → N2(g) + 2H2O(l)
The concentration of dissolved oxygen in a sample of water is 8.0 × 10−3 g\(\,\)dm−3.
Estimate the H−N−H bond angle in methanamine using VSEPR theory.
Ammonia reacts reversibly with water.
NH3(g) + H2O(l) \( \rightleftharpoons \) NH4+(aq) + OH−(aq)
Explain the effect of adding H+(aq) ions on the position of the equilibrium.
Hydrazine reacts with water in a similar way to ammonia. Deduce an equation for the reaction of hydrazine with water.
Outline, using an ionic equation, what is observed when magnesium powder is added to a solution of ammonium chloride.
Hydrazine has been used as a rocket fuel. The propulsion reaction occurs in several stages but the overall reaction is:
N2H4(l) → N2(g) + 2H2(g)
Suggest why this fuel is suitable for use at high altitudes.
Determine the enthalpy change of reaction, ΔH, in kJ, when 1.00 mol of gaseous hydrazine decomposes to its elements. Use bond enthalpy values in section 11 of the data booklet.
N2H4(g) → N2(g) + 2H2(g)
The standard enthalpy of formation of N2H4(l) is +50.6 kJ\(\,\)mol−1. Calculate the enthalpy of vaporization, ΔHvap, of hydrazine in kJ\(\,\)mol−1.
N2H4(l) → N2H4(g)
(If you did not get an answer to (f), use −85 kJ but this is not the correct answer.)
Calculate, showing your working, the mass of hydrazine needed to remove all the dissolved oxygen from 1000 dm3 of the sample.
Calculate the volume, in dm3, of nitrogen formed under SATP conditions. (The volume of 1 mol of gas = 24.8 dm3 at SATP.)
Many reactions are in a state of equilibrium.
The equations for two acid-base reactions are given below.
HCO3– (aq) + H2O (l) \( \rightleftharpoons \) H2CO3 (aq) + OH– (aq)
HCO3– (aq) + H2O (l) \( \rightleftharpoons \) CO32– (aq) + H3O+ (aq)
The following reaction was allowed to reach equilibrium at 761 K.
H2 (g) + I2 (g) \( \rightleftharpoons \) 2HI (g) ΔHθ < 0
Outline the effect, if any, of each of the following changes on the position of equilibrium, giving a reason in each case.
Identify two different amphiprotic species in the above reactions.
State what is meant by the term conjugate base.
State the conjugate base of the hydroxide ion, OH–.
A student working in the laboratory classified HNO3, H2SO4, H3PO4 and HClO4 as acids based on their pH. He hypothesized that “all acids contain oxygen and hydrogen”.
Evaluate his hypothesis.
Phosgene, COCl2, is usually produced by the reaction between carbon monoxide and chlorine according to the equation:
(i) Deduce the equilibrium constant expression, Kc, for this reaction.
(ii) State the effect of an increase in the total pressure on the equilibrium constant, Kc.
(i) Sketch the potential energy profile for the synthesis of phosgene, using the axes given, indicating both the enthalpy of reaction and activation energy.
(ii) This reaction is normally carried out using a catalyst. Draw a dotted line labelled “Catalysed” on the diagram above to indicate the effect of the catalyst.
(iii) Sketch and label a second Maxwell–Boltzmann energy distribution curve representing the same system but at a higher temperature, Thigher.
(iv) Explain why an increase in temperature increases the rate of this reaction.